Laboratory-derived numerical water quality criteria for copper, developed by the U.S. Environmental Protection Agency (EPA) in 1984 and updated in 1993, assume that the toxic form of dissolved copper exists in biologically treated effluents when, in fact, it does not. This causes erroneous permits to be issued, municipal resources to be misdirected, and industrial facilities to be adversely affected.
EPA's copper criteria should not be applied directly to municipal effluents for the following reasons:
These studies demonstrate that biologically treated effluents eliminate copper toxicity with significant additional complexing capability in reserve. Copper typically discharged (40 to 200 ug/L) by publicly owned treatment works (POTWs) should pose no threat to aquatic species. Laboratory research on the detoxifying effects of organic and inorganic matter on copper (including total organic carbon; particulate matter; and humic, fulvic, and amino acids) explains why scientific field studies consistently show that copper in biologically treated effluents is not expected to be toxic to aquatic life.
Impacts of Outdated Science
POTWs faced with inappropriate copper limits typically pass these limits on to the facilities' industrial users or modify potable water chemistry to reduce copper pipe dissolution. Certain industries also face inappropriate copper limits. Circuit board and textile manufacturers, for example, typically discharge organically complexed copper, which is nontoxic. Potable water suppliers can modify treatment to make their water less corrosive to copper pipe or eliminate use of copper sulfate (an algicide); however, these efforts may compromise drinking water quality and cause increases in other pollutants.
Such states as Connecticut, North Carolina, and Minnesota have started to respond to the inappropriate copper limits by avoiding application of EPA's recommended copper water quality criteria unless effluent toxicity to copper-sensitive organisms is indicated through routine effluent biomonitoring. EPA's Independent Applicability Policy, which suggests that states must use the most restrictive environmental indicator regardless of actual need, unfortunately bars the agency from supporting such action.
Regulatory Background
EPA's guidance for Clean Water Act (CWA) Sec. 304( a) criteria development requires that all relevant pollutant toxicity factors be considered in establishing water quality criteria for a specific pollutant. Because copper criteria are based on assessments of dissolved metal salts in laboratory water (with little or no ability to complex copper), the commonly encountered detoxifying effects of treated effluent and other naturally occurring substances were not considered. While this procedure may assess the maximum toxicological threat from copper, it does little to reflect real-world concerns.
Recognizing this flaw in the criteria document, EPA's 1993 guidance on implementing metals criteria says that only the bioavailable fraction of a metal should be regulated. Although recent guidance from EPA indicates that metals criteria assessed as "dissolved" may be a better approximation of the toxic fraction under some circumstances, measurements of filterable "dissolved" copper in biologically treated effluents rarely are relevant to assessing the bioavailable fraction of copper. Effluents with elevated dissolved copper routinely pass acute whole effluent and chronic toxicity tests using Daphnia species at copper levels much greater than the lethal concentration at which 50% of the test organisms die (LC50). In these cases, dissolved copper measurements erroneously assess nontoxic, filterable organo-copper complexes - the form in which the metal will be discharged from these facilities as dissolved. Because most facilities that discharge copper use biological treatment, it is apparent that widespread misapplication of the copper criteria results from use of a dissolved metals approach.
- The database used to derive existing criteria did not take into account copper detoxification by constituents commonly present in biological waste treatment systems
- Laboratory studies, field surveys, and water effects ratio procedures conducted by regulatory authorities and independent researchers since 1976 verify that copper rapidly binds with organic and inorganic matter during biological waste treatment, making it unavailable to interact biologically (nonbioavailable) and thus nontoxic to aquatic life.
- All EPA and state agency field studies confirm that copper in biologically treated effluents is not toxic to Daphnia, the sensitive species used to establish the federal copper criteria.
These studies demonstrate that biologically treated effluents eliminate copper toxicity with significant additional complexing capability in reserve. Copper typically discharged (40 to 200 ug/L) by publicly owned treatment works (POTWs) should pose no threat to aquatic species. Laboratory research on the detoxifying effects of organic and inorganic matter on copper (including total organic carbon; particulate matter; and humic, fulvic, and amino acids) explains why scientific field studies consistently show that copper in biologically treated effluents is not expected to be toxic to aquatic life.
Impacts of Outdated Science
POTWs faced with inappropriate copper limits typically pass these limits on to the facilities' industrial users or modify potable water chemistry to reduce copper pipe dissolution. Certain industries also face inappropriate copper limits. Circuit board and textile manufacturers, for example, typically discharge organically complexed copper, which is nontoxic. Potable water suppliers can modify treatment to make their water less corrosive to copper pipe or eliminate use of copper sulfate (an algicide); however, these efforts may compromise drinking water quality and cause increases in other pollutants.
Such states as Connecticut, North Carolina, and Minnesota have started to respond to the inappropriate copper limits by avoiding application of EPA's recommended copper water quality criteria unless effluent toxicity to copper-sensitive organisms is indicated through routine effluent biomonitoring. EPA's Independent Applicability Policy, which suggests that states must use the most restrictive environmental indicator regardless of actual need, unfortunately bars the agency from supporting such action.
Regulatory Background
EPA's guidance for Clean Water Act (CWA) Sec. 304( a) criteria development requires that all relevant pollutant toxicity factors be considered in establishing water quality criteria for a specific pollutant. Because copper criteria are based on assessments of dissolved metal salts in laboratory water (with little or no ability to complex copper), the commonly encountered detoxifying effects of treated effluent and other naturally occurring substances were not considered. While this procedure may assess the maximum toxicological threat from copper, it does little to reflect real-world concerns.
Recognizing this flaw in the criteria document, EPA's 1993 guidance on implementing metals criteria says that only the bioavailable fraction of a metal should be regulated. Although recent guidance from EPA indicates that metals criteria assessed as "dissolved" may be a better approximation of the toxic fraction under some circumstances, measurements of filterable "dissolved" copper in biologically treated effluents rarely are relevant to assessing the bioavailable fraction of copper. Effluents with elevated dissolved copper routinely pass acute whole effluent and chronic toxicity tests using Daphnia species at copper levels much greater than the lethal concentration at which 50% of the test organisms die (LC50). In these cases, dissolved copper measurements erroneously assess nontoxic, filterable organo-copper complexes - the form in which the metal will be discharged from these facilities as dissolved. Because most facilities that discharge copper use biological treatment, it is apparent that widespread misapplication of the copper criteria results from use of a dissolved metals approach.
Criteria Based on a Dissolved Metal
EPA has attempted to address concerns regarding proper application of metals criteria for the past 5 years. On May 28, 1992, in response to a petition for rulemaking, EPA released the Interim Guidance on Interpretation and Implementation of Aquatic Life Criteria for Metals, a final policy that modified all prior Sec. 304(a) criteria documents for metals. In issuing the interim guidance, EPA acknowledged that only the biologically available fraction of metals is responsible for aquatic toxicity and therefore is the proper focus of permit limit derivation: "The principal issue is the correlation between metals that are measured and metals that are biologically available," according to the Interim Guidance.
In the guidance document and related correspondence, EPA acknowledged that expressing water quality criteria for metals as dissolved measurements is a conservative approach and that states should consider further reductions in toxicity from complexing. Then-EPA assistant administrator for water, LaJuana S. Wilcher, wrote to Rep. John Paul Hammerschmidt (D-Ark.) in 1992, explaining that EPA was "allowing states to apply criteria to dissolved metals only," but the agency suspected that "this may be a somewhat less accurate method of excluding 'nontoxic' metal from regulation, because some dissolved metal exists in forms that have little toxicity ... " (particularly copper, a pollutant of great concern for municipal dischargers).
Following a January 1993 scientific conference in Annapolis, Md., on the development and implementation of metals criteria, EPA modified its criteria implementation guidance to use dissolved metal (filterable through a 0.45 µm membrane) concentrations in setting water quality standards (see Technical Guidance on Interpretation and Implementation of Aquatic Life Metals Criteria, EPA 1993). However, the scientists at the conference emphasized that, for highly reactive metals such as copper, dissolved metal standards may overstate the toxic fraction.
While these guidance documents were developed to avoid inappropriately stringent metals limitations, that objective has not been achieved for copper, because the preponderance of copper discharged by POTWs is in a dissolved form due to complexing with dissolved materials.
Since the Annapolis conference, EPA repeatedly has recognized that the dissolved metals approach for copper is unduly conservative; however, action has not been taken to ensure proper criteria application. For example, in a 1994 letter to the Pennsylvania League of Cities and Municipalities, EPA headquarters stated that "the organic matter and suspended solids normally present in both untreated and treated municipal wastewater have a substantial effect in binding metals and reducing bioavailability, particularly for copper. Thus, we ordinarily expect copper discharged by municipalities, or in the presence of municipal effluent, to have less toxicity per unit concentration than would soluble copper salts added to clean natural waters. We agree, that if these national criteria are applied in situations with high organic matter and suspended solids, that the level of protection would increase above that determined to be minimally necessary in the national criteria." This recognition was reiterated in a subsequent letter from EPA Region 2 to the New Jersey Department of Environmental Protection.
Unfortunately, because the dissolved metals approach equates all "filterable" dissolved copper to bioavaiIable copper, permittees still routinely receive stringent copper limitations where there clearly is no environmental need. Sufficient laboratory and field results exist at this time to warrant a correction to this misapplication of the copper criteria.
Copper Detoxification Studies
Numerous studies verify that copper is particularly amenable to complexation with organic and inorganic matter to render this metal nonbioavailable. The detoxifying influence of organic and inorganic complexation on copper was reported in EPA's 1984 Copper Criteria Document. For copper, aquatic organisms respond to free ionic metal and monohydroxy complexes as bioavailable forms. The criteria document acknowledged that rapid detoxification of copper in the presence of inorganic and organic substances occurs due to the metal's high reactivity.
EPA's criteria application guidance provided a criteria adjustment for hardness but omitted similar consideration of organic ligands, even though the agency recognized their greater importance in detoxifying copper. For example, the copper criteria presented studies that evaluated the toxicity of copper to Daphnia pulicaria in various surface waters and found that total organic carbon (TOC, an indicator of organic ligand concentration) is a more important variable affecting toxicity than hardness, with acute values varying approximately 30-fold over the range of TOC covered. Similar results were obtained with the fathead minnow. Accordingly, the criteria acknowledged that it should be adjusted upward for surface waters with TOC significantly above the 2 to 3 mg/L typically found in waters used for toxicity tests.
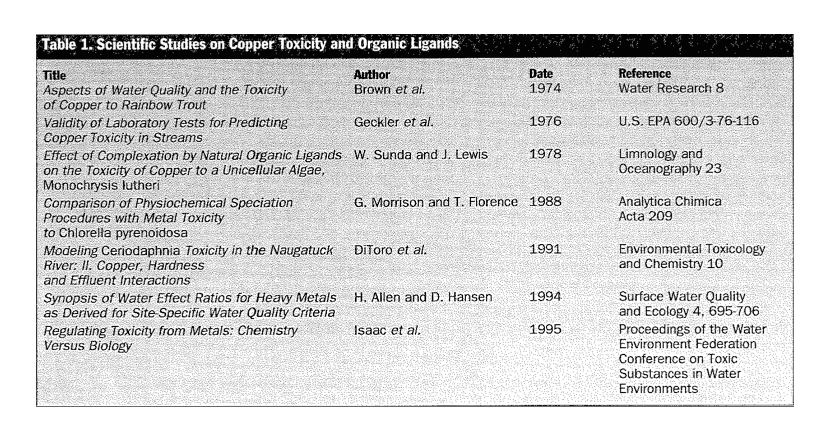
The scientific literature is replete with peer-reviewed studies confirming that organic ligands similar to those in municipal effluents mitigate copper toxicity (see Table 1).
Laboratory studies conducted under conditions with relatively low levels of binding agents confirm that even when relatively high "dissolved" copper concentrations were measured, the toxicity of copper to sensitive species was greatly reduced or eliminated in the presence of organic and inorganic compounds. As the amount of ligands and other binding agents is, stoichiometrically, in excess of the ionic copper for typical municipal conditions, for all practical purposes, no toxic copper will be present. This fact was demonstrated by H.E. Allen and D.J. Hansen at the University of Delaware in Newark in January 1994 using standard analytical techniques for quantifying binding agents of mixtures.
On the basis of more than 20 years of observations and research on metal speciation chemistry and fate of metals in receiving waters and treatment facilities, Allen, a nationally recognized expert on metals toxicity, concluded that virtually all copper in a municipal treatment plant effluent will be in the form of soluble copper complexes or sorbed to particulate material not removed from the effluent stream in the final clarifier. The effluent also will contain a finite concentration of free, ionic copper, but this low concentration will not pose a toxicity risk.
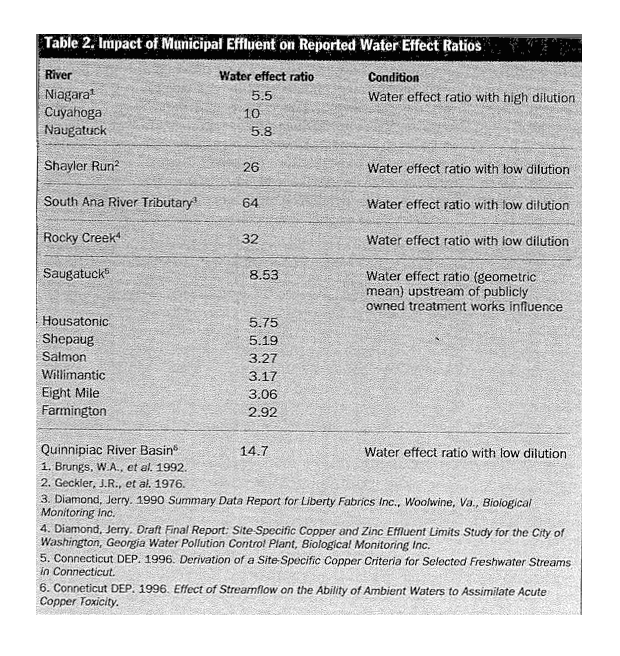
Field studies of water effect ratios, which add metal salts to effluents in an attempt to gauge potential toxicity, have repeatedly confirmed laboratory observations and validate the total detoxification of copper by biologically treated effluents (see Table 2). For example, in January 1991, DiToro et al. performed water-effect ratios on the site-specific detoxification of copper in the Naugatuck River in Connecticut. Very little difference in toxicity was observed between laboratory water with minimal complexing ability and river water from pristine segments. However, where river water contained treated municipal effluents, up to a 12-fold reduction in copper toxicity was recorded. The-study team concluded that the copper present in the municipal effluent was nontoxic. Moreover, the municipal effluents contained excess binding capacity that rendered bioavailable copper from upstream sources nontoxic.
A 1992 EPA-funded summary of water effect ratios for heavy metals compiled by William Brungs showed that copper is up to 26 times less toxic in water influenced by municipal effluent. To have a water effect ratio significantly above 1.0, the existing metal in the discharge must be complexed. The water effect ratio actually represents the excess binding capacity of the effluent. In general, if a water effect ratio is greater than 2 or 3, the effluent metal should be classified as nontoxic.
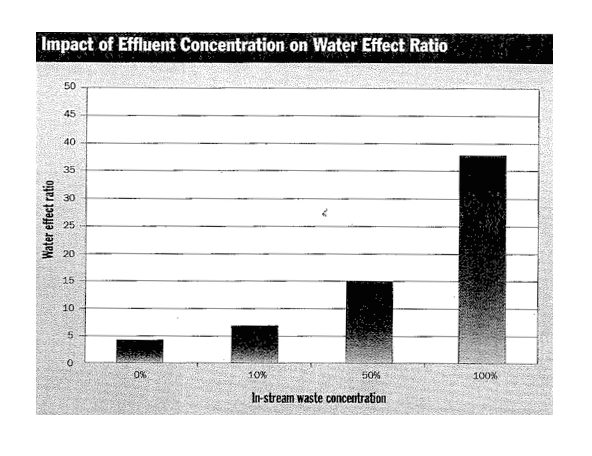
Most recently, testing by the Connecticut Department of Environmental Protection confirmed that copper toxicity was significantly reduced in ambient river water above municipal discharges, with water effect ratios ranging from 3 to 5. The state agency further found that when ambient river water was mixed with treated municipal wastewater effluent, the water effect ratio typically exceeded 10 at effluent concentrations greater than 20% (see Figure 3). Connecticut has amended the state copper water quality standards for all receiving waters with a high domestic wastewater component (greater than 20%) by applying a minimum water effect ratio of 3 to avoid over-regulation.
These field results confirm observations made by laboratory researchers and validate the complete detoxification of copper in the presence of biologically treated effluents. There are no reported instances in which copper in biologically treated effluent was toxic to the sensitive species EPA used to set its criteria. Thus, the dissolved copper criteria approach for biologically treated effluents wastes local resources on problems that do not exist.
Proper Application of Science
The fundamental oversight in translating dissolved copper criteria into permit conditions is the failure to regulate only the bioavailable metal. The laboratory conditions of EPA's criteria development experiments accurately reflect the maximum toxic impacts to highly sensitive species when exposed to a highly toxic, dissolved, ionic copper in pure water having little or no complexing ability. Such conditions are unrelated to copper discharged from biological waste treatment systems. This is particularly true for effluent-dominated, low dilution streams where proper criteria application is most critical. Federal water effect ratio procedures are unnecessary for this class of dischargers.
The language of EPA regulations makes it clear that the agency's authority to develop criteria rests on the scientific accuracy by which those criteria relate to aquatic impacts: "Sec. 304(a) criteria are developed by EPA under authority of Section 304(a) of the [CWA] act based on the latest scientific information on the relationship that the effect of a constituent concentration has on a particular aquatic species and/or human health."
Integration of analytical and biological test results could avoid the need for expensive and time-consuming water effect ratio procedures. By allowing scientifically defensible biomonitoring-bioassay methods as an alternative method of assessing water quality criteria compliance and developing water quality-based effluent limitations, adequate protection from the toxic or bioavailable fraction of copper would be ensured. The use of bioassay tests with copper-sensitive organisms to directly evaluate the bioavailable fraction of copper is related to the actual potential for aquatic life effects to Daphnia. Consequently, acute whole effluent toxicity testing using daphnids should be accepted as the basis for confirming that water quality standards are being maintained, regardless of the amount of copper present in a discharge.
The North· Carolina "action level" approach exemplifies a more reasoned evaluation of copper. The state recognizes that the level of metals toxicity is variable and depends on chemical form, solubility, stream characteristics or associated waste characteristics and has established dissolved "action levels" for metals, including copper. Exceeding a dissolved copper action level in-stream triggers an evaluation of whether the effluent is acutely toxic and whether the toxicity is attributable to copper. If sufficient evidence exists to confirm that effluent toxicity is caused by copper, then a copper limit is imposed.
Application of water quality standards for copper must reflect real-world impacts. The National Guidelines require revision of criteria whenever they are found to be "substantially over- or under protective." As the dissolved metals approach for copper has been demonstrated to be overprotective in all cases involving biologically treated effluents, this guidance document requires revision of EPA's general approach for regulating copper and reconsideration of the Independent Applicability Policy.